
| HOME |
| LEADERSHIP |
| SERVICES |
| PUBLIC SEMINARS |
| REGISTER |
| ARTICLES |
| SITE MAP |
| CONTACT |
| ESPAÑOL |
Statistically Sound Product and Process Validation for World Class Productivity and Quality

Convert your validation process from a necessary evil to an asset which assures you success in meeting productivity and quality goals. The ZDM Group offers a progressive and comprehensive approach to process and product validation derived from our work in implementation of Six Sigma manufacturing systems and turn-key engineering projects for the beverage, packaging and plastics industries. We can supplement your company's validation and compliance staff or provide complete turn-key validation projects.
Our strategic alliance with Thomas King Consulting, Inc. brings expertise in document control, related to FDA's Quality System Regulation, ISO 9000/13845 Quality System, Medical Device Directive, ISO 14971 Risk Management, ISO 14001 Environmental Management, and other related standards as well as the National Baldrige Quality Program.
The validation process:
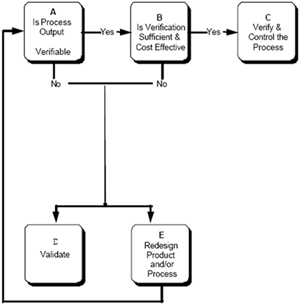
Services include:
- Implementation of statistically-based equipment, process, and product qualification and validation including Operational Qualification (OQ) and Performance Qualification (PQ)
- Education in:
- Functional Analysis
- Problem Solving Tools
- Basic Statistics
- Statistical Process Control (SPC)
- Process and Quality Measurement Precision and Reliability (Gauge R&R)
- Statistical Design and Analysis of Experiments (DOE)
- ANOVA, regression, hypothesis testing, box and whisker, confidence interval plots
- Reporting methods
- SOP Documentation
- Performance Measurement
